Book An Appointment
HCD Blog
For the latest in Skin, Science and Beauty
FDA Approves Kybella for Treatment of Submental Fullness
By Humphrey Cosmedic Dermatology on April 30th.
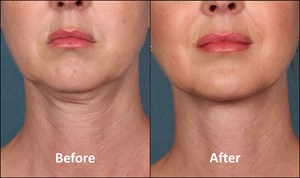
On April 29, 2015, the U.S. Food & Drug Administration approved Kybella (also known as ATX-101) – Kythera Biopharmaceuticals Inc.’s injectable drug for adult submental fat, the first nonsurgical option for treating the double chin.
Dr. Shannon Humphrey and Dr. Jean Carruthers were both principal investigators in the clinical trials that ultimately resulted in the approval of this exciting new treatment for submental fullness due to submental fat.
As experts on Kybella, they presented the trial data at a number of international meetings and, as part of the national launch in the US, will use their in-depth knowledge of the product to train fellow physicians on proper injection techniques.
What is ‘submental fullness’?
Often referred to as a “double chin,” submental fullness is a common complaint for patients but, until now, there have been limited options for treatment. The condition can be caused by aging, genetics or weight gain. According to a 2014 survey by the American Society for Dermatologic Surgery (ASDS), over two-thirds of people said they are bothered by submental fullness.
Although Kybella is still under consideration by Health Canada for approval, patients interested in finding out more in advance of possible treatment can contact Carruthers & Humphrey at 604-714-0222 to make an appointment for consultation.
Photo source: KYTHERA Biopharmaceuticals, Inc.
Blog Categories
- All
- Acne
- anti-aging
- Antiaging
- Beauty
- Beauty Expert
- Belkyra
- Botox
- Carruthers & Humphrey
- Clinical Research
- Complexion
- Cosmetic Dermatology
- Cosmetic Procedures
- Dr Jean Carruthers
- Dr Katie Beleznay
- Dr Shannon Humphrey
- HA Filler
- IPL
- Positive Aging
- Skincare
- Skinmedica
- SPF
- Sun protection
- Thermage
- vancouver
- Wrinkles
